760 mm Hg 137 mm Hg 623 mm Hg. 12 12 Which measurement describes the pressure of a gas.

5 2 Gas Pressure And Its Measurement Chemistry Libretexts
Device used to measure the pressure of a gas trapped in a container pascal Pa SI unit of pressure.

. 3 Show answers Another question on Chemistry. E 045 moles Answer. 450 mm Hg denotes the air pressure.
What is the pressure of this gas in atmospheres and torr. 760 mm Hg 137 mm Hg 623 mm Hg. Pressure The pressure of a gas is defined as the force exerted per unit area by a gas against or.
In mm Hg this is. There are 500 moles of gas molecules in a container. The pressure of the gas equals the hydrostatic pressure due to the pressure of the atmosphere at sea level minus a column of mercury of height 137 cm.
Which measurement describes the pressure of a gas. Put another way P1V1 P2V2. Since the gas in your graduated cylinder is a mixture of butane and water vapor you must determine the partial pressure.
No of moles n 500 mol temperature T 220 K Volume V 40 L. Chemistry questions and answers. One pascal equals one newton per square metre.
In the kinetic molecular theory of gas behavior the distance between gas molecules is assumed to be _____ the diameter of the gas. Which measurement describes the pressure of a gas. Why is silver chloride is an ionic compound.
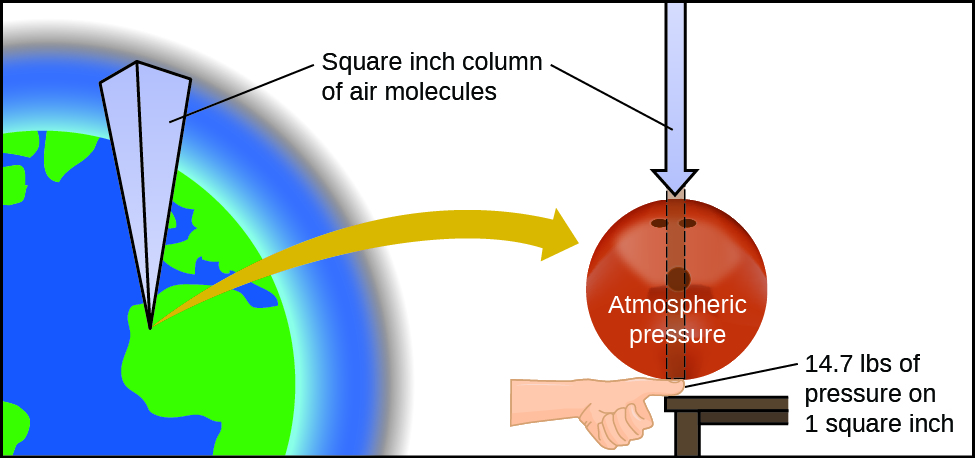
GL is a unit for density. Atmospheric pressure is close to 100000 pascals. The waves used in radar systems are _____ waves.
Which measurement describes the pressure of a gas. A 450 mm Hg Explanation. A mm Hg b atm c kPa.
25L is a value for volume. Which measurement describes the pressure of a gas. I hope this was helpful.
D None of the above. Solution- D725 mmha gt desc nibes the Anessure t a 3as の4カon 13 333 223 87 y 15 Pa. In the United States where the System International SI is still just a fad we can still find pressure in pounds per square inch or psi.
1 atm 760 mmHg or 1 atm 760 torr torrcellis In physics the measure is completed in units called Pascals where 1 atm of gas is equivalent to 101325 Pa or 1013 kPa kiloPascals. Subsequently question is how a manometer is used to measure gas pressure. Boyles law describes the relationship between volume of a gas and the pressure of that gas when the temperature remains constant.
However the SI unit for pressure is the pascal Pa which equals approximately 1 kg m-1 s-2. According to the kinetic theory of gasesa gas can be compressed much more than a liquid or solid be. Which of the following measurement describe the pressure of a gas.
A 264 cm Hg 10 mm Hg 1 cm Hg 1 torr 1 mm Hg 264 torr. The pressure on the left is due to the gas and the pressure on the right is due to the atmospheric pressure minus 137 cm Hg. October 21 2021 thanh.
315K is a value for Asbolute T Kelvin grade Advertisement. Which measurement describes the pressure of a gas. The pressure of the gas equals the hydrostatic pressure due to the pressure of the atmosphere at sea level minus a column of mercury of height 137 cm.
- 14846962 sadgirl928 sadgirl928 02202020 Chemistry High School answered Which measurement describes the pressure of a gas. A medical laboratory catalog describes the pressure in a cylinder of a gas as 1482 MPa. If 220 K temperature is applied to the gas of a volume of 40 L identify the Gas pressure.
Analyst Bank Clerk Bank PO. AIEEE Bank Exams CAT Job Role. MmHg is a sort of unit to determine pressure.
A 045 moles B 725 mmHg C 25 L D 12 gL E 315 K. 1 Pa 1 Nm 2 pounds per square inch psi unit of pressure common in the US. The pressure on the left is due to the gas and the pressure on the right is due to 264 cm Hg or mercury We could use the equation p hρg as in Example 92 but it is simpler to just convert between units using Table 91.
What measurement describes pressure. A315 k B12 gl C725 mm Hg 4. A 045 moles b 315 K c 12 gL d 25 L e 725 mmHg.
A 450 mm Hg. The pressure on the left is due to the gas and the pressure on the right is due to the atmospheric pressure minus 137 cm Hg. One atm equals 101325 x 10 5 Pa.
The Gas pressure formula is given by P 500 0. A manometer is a device similar to a barometer that can be used to measure the pressure of a gas trapped in a container. In SI units pressure is measured in pascals.
The pressure of a sample of gas is measured at sea level with an open-end Hg mercury manometer as shown to the right. Determine the pressure of the gas in. Which measurement describes the amount of a gas.
A 315 gL b 2456 mbar c 255K d 50 grams. The SI unit for Gas pressure is expressed in Pascals Pa. For a gaswhich pair of variable are inversely proportional to each other if all other conditions r.
This means that as the pressure is decreased the volume of a fixed amount of gas will _____ provided the temperature is kept constant. A 315 K b 12 gL c 25 L d 725 mmHg e 045 moles. 12 12 Which measurement describes the pressure of a gas.
In mm Hg this is. Which measurement describes the pressure of a gas. Solution The pressure of the gas equals the hydrostatic pressure due to a column of mercury of height 137 cm plus the pressure of the atmosphere at sea level.
Which measurement describes the pressure of a gas. Which measurement describes the pressure of a gas. What measurement describes the pressure of gas.
The volume of a gas is _____ related to its pressure.

Boyle S Law Boyle S Law Describes The Relationship Between The Pressure And Volume Boyle S Law Gas Laws Chemistry Physical Chemistry

Gas Laws Formula Of Universal Gas Law

Relating Pressure Volume Amount And Temperature The Ideal Gas Law Chemistry For Majors
0 Comments